Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Evaluation of Sperm DNA Fragmentation in Infertile Men with Varicocele
*Corresponding author: Fabio Scarpellini, Cerm Hungaria, Roma, Italy.
Received: March 14, 2023; Published: March 30, 2023
DOI: 10.34297/AJBSR.2023.18.002470
Abstract
Although the pathophysiology of the testicular damage associated with varicocele remains unclear, sperm DNA damage has been identified as a potential explanation for this cause of male infertility. The current study was designed to determine the extent of sperm nuclear DNA damage in patients with varicocele, and to examine its relationship with parameters of seminal motility.
Semen samples from 50 patients with clinical varicocele and 30 infertile men without varicocele were examined. Varicocele sperm samples were classified as normal or pathological according to the 1999 World Health Organization guidelines. Sperm DNA damage was evaluated using the Halo sperm kit, an improved Sperm Chromatin Dispersion (SCD) test.
The DNA fragmentation index (DFI: percentage of sperm with denatured nuclei) values was significantly higher in patients with varicocele, either with normal or abnormal (DFI 23.5±3.2 vs 13.4±2.4-P<0,01) semen profiles.
Varicocele is associated with high levels of DNA-fragmentation in spermatozoa. In addition, in subjects with varicocele, abnormal spermatozoa motility is associated with higher levels of sperm DNA fragmentation. DNA fragmentation may therefore be an essential additional diagnostic test that should be recommended for patients with clinical varicocele.
Introduction
Varicocele is one of the most frequent causes of male infertility [1,2,3,4,5] with a prevalence in adolescents.
It consists of a dilatation of the pampiniform venous plexus that overlies and surrounds the testicle and is probably one of the most common causes of oligoasthenozoospermia. However, the pathogenetic mechanisms through which varicocele induces testicular dysfunction with consequent alteration of spermatogenesis have not yet been fully clarified. Several factors are involved: venous stasis with consequent testicular hypoxia and increase in temperature inside the testis with consequent altered production of spermatogenic cells by the germinal epithelium, damage to the hypothalamic-gonadal axis or Stress Oxidative (OS) [6] Meta-analysis studies on infertile patients with a clinical diagnosis of varicocele have identified oxidative stress as one of the major causes of sperm dysfunction with a negative impact mainly on the plasma membranes which results in a drastic reduction in sperm motility, as well as in the fluidity and integrity of membrane. Furthermore, it has been seen that infertile patients with clinical varicocele almost always presented a high degree of sperm DNA fragmentation [7] compared to other types of infertile, suggesting a possible correlation between ROS production and increased fragmentation of sperm nuclei. In this study, the fragmentation of sperm DNA was determined in infertile subjects diagnosed with varicocele, compared to a sample of fertile and normozoospermic subjects.
Materials and Methods
80 subjects referred to our clinic for andrological problems were included in the study. From a clinical examination and instrumental investigations (color Doppler ultrasound and testicular ultrasound) it was possible to divide the patients into two subgroups of infertile: 30 with varicocele of various degrees, 20 with infertility of other nature. The control group included 30 healthy men, with normal genitalia and normal semen parameters according to WHO 2010. Subsequently, on the sperm population of each patient considered in the study, the analysis of sperm DNA fragmentation was carried out using the SCD method (Sperm Chromatin Dispersion) test contained in the Halo sperm kit which allowed to evaluate in each of them the percentage of sperm DNA fragmentation expressed in % DFI (Dna Fragmentation Index).
Sperm DNA Fragmentation Analysis
To determine the level of DNA fragmentation in spermatozoa, the Halosperm kit (Diasint-CGA, Florence) was used, in which the technique known as the SCD-Sperm Chromatin Dispersion test (Fernandez et al., J Androl.24: 59-66, 2003; Fertil Steril 84: 833- 842, 2005). This technique is based on the differential response of human sperm nuclei with fragmented DNA compared to those with intact DNA in which, a controlled denaturation of the DNA, followed by the extraction of the nuclear proteins, generates the so-called nuclides which represent the deproteinized nuclei of the sperm composed in turn from an internal part “the core” located in the center and from a peripheral halo due to the DNA loops that expand forming the so-called “chromatin dispersion halo” in the presence of DNA is not fragmented. Conversely, when fragmentation is present, nuclides do not develop the halo of dispersion or, if they do, minimally. In all of this, the sperm tail is visible and represents an important morphological parameter that allows the nuclei of other cells to be distinguished from the sperm nuclei in which the tail is obviously present. The fresh semen samples belonging to the patients we considered in the study were initially diluted in Phosphate Buffered Saline (PBS) at a concentration of 5 million per milliliter; 25 microliters of each of them were included and mixed in a previously dissolved agarose microgel (contained in the kit). Subsequently, about 20 microliters of this suspension of cells were taken from the agarose test tube and stratified on a pre-treated slide suitably covered with a coverslip and kept horizontally on a metal plate at 4°C for 5 minutes.
After this time, the slide with the suspension on it and freed from its coverslip, was immersed in a denatured solution for 7 minutes at room temperature and subsequently transferred into a tray containing 10 ml of lysing solution in which it was left to incubate for the duration of 25 minutes.
The next step consisted in removing the lysing solution by rinsing the slide in abundant distilled water leaving it to act for 5 minutes, after which the same slide was incubated for 2 minutes each in three solutions of ethanol at increasing concentration (70%, 90%, 100%) and left to dry at room temperature. The last step concerned the staining of the slide for reading and observation under a bright field microscope, using Wright’s staining solution.
Scoring Criteria
At least 500 spermatozoa per sample were studied, adopting
the following criterion which allowed us to distinguish two types
of spermatozoa:
a) spermatozoa without fragmented DNA, include
spermatozoa that have a “large halo”-with a thickness equal to
or greater than the length of the minor diameter of the nucleus
(core)-or “medium halo”-with a thickness greater than 1/3 of
the minor diameter of the nucleus (core).
b) Spermatozoa with fragmented DNA, include spermatozoa
that have a “small halo”, with a thickness equal to or less than
1/3 of the minor diameter of the nucleus and sometimes
even irregular in shape; o spermatozoa “without halo” and
with signs of deterioration where not only is there no halo,
but there are also evident signs of cellular suffering with the
nucleus fragmented into granules and very light staining (to
be distinguished from artifacts due to the staining technique
used)
Statistical Analysis
All data were reported as mean + standard deviation and statistically analyzed using the SPSS statistical program. The statistical tests used were for continuous values the student t’ test and for parametric values χ2.
Results
This work highlighted how the % of nemasperms with fragmented DNA of the examined patients, expressed in DNA Fragmentation Index (DFI) is significantly higher than in the infertile group (23.5+3.2 and 13.4+2.4 respectively: P<0.01). To corroborate the fact that the DFI of patients with varicocele is higher, the values collected in the study of infertile patients with varicocele and infertile for other causes were compared.
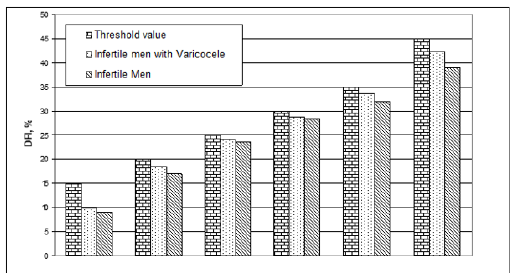
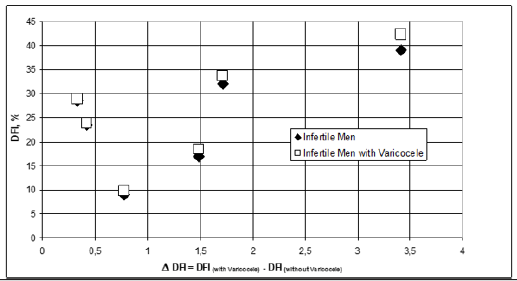
Subsequently the same values were grouped, for each category of patients considered, on the basis of threshold values, set at 15, 20, 25, 30, 35 and 45, corresponding to conditions ranging from normality to seriousness. Finally, the differences of these groups of mean values were evaluated, such as Varicocele DFI-infertile DFI. These differences have been correlated to the DFI values of the two categories and it is observed that the difference is always positive and higher for patients with varicocele (Figure1& Figure2).
Discussion
It is now known that varicocele negatively affects spermatogenesis, although the mechanisms are still not well understood. It has recently been hypothesized by several authors in the literature that sperm dysfunction in varicocele could be related to an increase in sperm DNA fragmentation levels. One of the causes of this phenomenon has been identified in the presence of high production of ROS in this type of patient, according to some authors found at significantly higher levels in subjects with clinical varicocele of 2nd and 3rd degree, which is related to an impaired motility sperm, quite frequent in subjects affected by this pathology. The ROS are responsible not only for the lipid peroxidation of the plasma membranes of the spermatozoa, but also for the alteration of the chromatin integrity of the same. A higher incidence of sperm DNA fragmentation present in infertile patients with varicocele compared to other types of infertile patients emerged from our study and only confirms the hypotheses up to now supported by the various authors [8-26]. The need to include in the clinical management of patients with varicocele, the study of fragmentation of sperm DNA with possible advice on the potential negative effects that the increase in the levels of damaged sperm DNA can induce on future fertility is growing.
Acknowledgement
None.
Conflict of Interest
None.
References
- Naughton CK, Ajay K Nanjia, Ashok Agarwal (2001) Varicocele and male infertility: Part II. Pathophysiology of varicoceles in male infertility. Hum Reprod 7(5): 473-481.
- Kursh ED (1987) What is the incidence of varicocele in a fertile population? Fertil Steril 48(3): 510-511.
- Sakkas D, E Mariethoz, G Manicardi, D Bizzaro, PG Bianchi, et al. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod 4(1): 31-37.
- Zini A, K Kamal, D Phang, J Willis, K Jarvi (2001) Biologic variability of sperm DNA denaturation in infertile men. Urology 58(2): 258-261.
- Sharma RK, FF Pasqualotto, DR Nelson, AJ Thomas Jr, A Agarwal (1999) The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod 14(11): 2801-2807.
- Hauser R, G Paz, A Botchan, L Yogev, H Yavetz (2001) Varicocele and male infertility: Part II. Varicocele: effect on sperm function. Hum Reprod Update 7(5): 482-485.
- Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79(4): 829-843.
- Barbieri ER, ME Hidalgo, A Venegas, R Smith, EA Lissi (1999) Varicocele-associated decrease in antioxidant defenses. J Androl 20(6): 713-717.
- Barroso G, Morshedi M, Oehninger S (2000) Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod 15(6): 1338-1344.
- Evenson DP, LK Jost, D Marshall, MJ Zinaman, E Clegg et al. (1999) Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 14(4): 1039-1049.
- Evenson DP, Kjersten L. Larson, Lorna K Jost (2002) Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl 23(1): 25-43.
- Hauser R et al. (2001) Varicocele: effect on sperm functions. Hum Reprod Update 7: 482-485.
- Irvine DS, JP Twigg, EL Gordon, N Fulton, PA Milne, et al. (2000) DNA integrity in human spermatozoa: relationships with semen quality. J Androl 21(1): 33-44.
- Pasqualotto FF, Sharma RK, Nelson DR, Thomas AJ, Agarwal A (2000) Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril 73(3): 459-464.
- Fernàndez JL, Lourdes Muriel, Vicente Goyanes, Enrique Segrelles, Jaime Gosálvez, et al. (2005) Simple determination of sperm DNA fragmentation with an improved sperm chromatin dispersion (SCD) test. Fertil Steril 84(4): 833-842.
- Fernàndez JL, Lourdes Muriel, Maria Teresa Rivero, Vicente Goyanes, Rosana Vazquez, et al. (2003) The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 24(1): 59-66.
- Hendin BN, PN Kolettis, RK Sharma, AJ Thomas Jr (1999) A Agarwal Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol 161(6): 1831-1834.
- Mitropoulos D, G Deliconstantinos, A Zervas, V Villiotou, C Dimopoulos, et al. (1996) Nitric oxide synthase and xanthine oxidase activities in the spermatic vein of patients with varicocele: a potential role for nitric oxide and peroxynitrite in sperm dysfunction. J Urol 156(6): 1952-1958.
- Zini A, Defreitas G, Freeman M, Hechter S, Jarvi K (2000) Varicocele is associated with abnormal retention of cytoplasmic droplets by human spermatozoa. Fertil Steril 74(3): 461-464.
- Naughton CK, Nangia AK, Agarwal A. Varicocele and male infertility: Part II.
- Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Agarwal A (1999) The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod 14(11): 2801-2807.
- Hauser R, Paz G, Botchan A, Yogev L, Yavetz H (2001) Varicocele: effect on sperm function. Hum Reprod Update 7:482-5.
- Barbieri ER, Hidalgo ME, Venegas A, Smith R, Lissi EA (1999) Varicocele-associated decrease in antioxidant defenses. J Androl 20(6): 713-717.
- Chen SS, Chang LS, Wei YH (2001) Oxidative damage proteins and decrease of antioxidant capacity in patients with varicocele. Free Rad Biol Med 30(11): 1328-1334.
- Villanueva Diaz CA, Vega Hernandez EA, Diaz Perez MA, Echavarria Sanchez M, Ortiz Ibarra FJ, et al. (1999) Sperm dysfunction in subfertile patients with varicocele and marginal semen analysis. Andrologia 31(5): 263-267.
- Saleh RA, Agarwal A, Nelson DR, Nada EA, EL Tonsy MH, et al. (2002) Increased sperm nuclear DNA damage in normozoospermici infertile men: a prospective study. Fertil Steril 78(2): 313-318.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.